A so called greynet of online pharmacies exists that offer medicines for sale without a prescription. People use these sources often because they’re cheaper or they don’t have access to adequate healthcare options. While it is popularly known that these pharmacies are questionable, it is seldom seriously discussed amongst the people who buy from them. Understanding regulation of the pharmaceutical industry and how it relates to consumer protection is important in protecting oneself in this situation. Using freely available online tools, it is possible to vet a manufacturer and form a risk assessment. The interpretation of plant inspections and other public data or the lack thereof can give insight by which to ascertain confidence and comfort with the idea of consuming a given product.
Isn’t this illegal? Are you really gonna teach people it’s alright to take drugs not appproved by their doctor?
I believe people have the right to put whatever they want into their body and that the government’s duty on this front is to provide support in the form of evidence based research to inform responsible choices and the establishment of adequate systems to promote a healthy life. The US fails at this in multiple regards, and I disagree with many existing policies. My intent is to fill part of this gap in providing guidance for the sufficiently motivated to conduct reseaerch and apply judgement aligned with their tolerance for risk in the interest of harm reduction. Other resources exist, for example, to educate trans people in basic endocrinology such that they may make informed decisions based on laboratory testing throughout their self-guided treatment and seek medical expertise if necessary. My work here is a companion to this and similar efforts.
Yes, this is technically illegal. Will you get in trouble with the law for it, though? No. The FDA in conjunction with the US Postmaster and Customs does not enforce against (read: prosecute) the importation of non-scheduled medicines for personal use. Your parcel might be seized and you issued a letter from USPS to discourage the practice or inform you of the drug being unsafe or requiring medical supervision, but as long as the quantities shipped are reasonable, that’s the end of the story. Surprisingly, most of this is even printed in their inspections manual on imports (link to manual below):


That said, don’t blame me if after reading this, you take some medication you found online and something unexpected happens. As will be clearly shown in some cases, there is some risk involved and we cannot with certaintly quantify all of it due to unknowns. Even if we could, it would express to you that there is some probability of harm. This is independent of negative reactions from the actual active ingredent. In the same vein, I am not explicitly recommending that anyone personally purchase and consume these products, sponsoring them or their suppliers.
Legal pharmacies sell this stuff in other countries! What’s the big deal?
While greynet pharmacies likely do not have a significant risk of adulteration or tampering of products sold once they arrive in the business’s hands, they do suffer from quality control issues at a local and manufacturer level. The former may result in suboptimal storage conditions resulting in degradation of product. The latter relates to sourcing products from countries lenient with exports. These countries may have no published inspections data conducted by authorities within the country of origin, not be sold in countries that do publish inspections, or have higher inspection violation rate than others when external authorities investigate. Some examples include India, and Turkey, which suffer from each of these problems, as the public is frequently warned about by regulatory authorities.
Inspections of manufacturer sites are important to check for compliance with standardized Good Manufacturing Practices. Failure to comply stringently with these practices can result in unintended adulteration of product, leading to customer complaints, adverse reactions, or severe health effects. Examples of adulteration or what can lead to it include:
- active ingredients deviating from intended tolerances
- contamination with bio-organisms or inorganic substances
- systemic problems in QA
- failure to test batches, do so appropriately, or act on the result of testing
- problems in storage of batches
- equipment not maintained for standards of cleanliness in production
- inadequare record keeping, failing to respond to complaints, etc
Many regulatory authorities such as the US Food and Drug Administration, and European Medicines Agency conduct inspections of manufacturing sites worldwide pursuant to applications from companies to sell their products within the countries for which the compiance standards and regulations exist to the benefit of. Publically searchable databases are made available by these agencies and as such, we can leverage them to do research on products offered at greynet pharmacies and attempt to inform ourselves of their risks before purchase.
How does one form a risk assessment of a product?
In the example below, I will walk you through several specific products and my methodology for evaluating the manufacturer. In summary, these are the steps I take. They are not comprehensive, and some investigations require rather vigorous exploration of alternate research avenues.
- Determine the manufacturer and the medicine’s country of origin
- Search for registries of the product with local regulatory authorities’ databases
- Investigate inspections records for plants known or suspected of being involved in production
- If there are violations, assess severity of them with respect to health hazard or confidence in the manufacturer’s practices
- If there is not enough information, seek out alternate research avenues
- Contexualize your findings within the bounds of your tolerance for risk to determine comfort
There are many reliable tactics one can use during this research, but the methods outlined here are not a comprehensive and complete list. It may be impossible to know which specific site(s) a drug of interest is produced at. Without a drug registration document at hand to tell us this, there is some level of unknown risk. Be mindful of the possibility that there exists some site that hasn’t been inspected since its formulations are not exported to the EU or US, even if there are others that do. To work around this, the liklihood of this can be assessed by familiarizing yourself with the company’s strategy, such as global presence or product availability and marketing reach.
Your best aid in a complex assessment is going to be a search engine. Learn as much as you can about the company and the product you’re interested in from their website(s), news articles, government filings, etc. You don’t need to know how the pharmaceutical and manufacturing industries work before undertaking this effort to learn and make inferrences. Critical thinking and general research skills will be your best friend. If you’re considering taking medical decisions into your own hands, these are essentials skills to nurture.
Tools
- Search engine
- Manufacturer’s website
- PharmacyCompass - aggregator of manufacturers, inspections, registrations, etc
- EudraGMDP - European database for GMP inspections
- Country specific GMP regulatory authorities and databases, such as Medsafe in New Zealand
- US Food and Drug Administration
- Wikileaks
Regulatory Information
- US FDA
- Inspections Database FAQ
- Regulatory Procedures Manual, Ch 9. Import Operations and Actions
- Compliance Program Guance Manual
- Drug Manufacturing Inpsections (Part III Inspectional and Part V Regulatory/Administrative Strategy)
Whomst might buy from the greynet?
Let’s say I’m a trans girl who wants to start HRT. I live in the US, and there are no informed consent clinics in my area. People are quite conservative, and there’s no way any doctor would help me out, even if I were to obtain a GID referral letter somehow. I’m of legal age, and have the money to afford the medicine plus a bank account to make the purchase. It’s a familiar story. I decide to DIY, so I take to the net and see what I can buy on my own.
A simple example
Consider Procalut/Casodex (Bicalutamide). I heard its a good antiandrogen with few if any side effects on average. It’s sold by a couple pharmacies (UP, 4nrx) at the cheaper price point than other commonly recommended ones.
Both list the manufacturer and country of origin. Good! Other pharmacies might not (looking your way, IHP). This is important information if the manufacturer has multiple plants spread the globe. Look up the supplier to get an idea if this is the case. For this product, that’d be Kocak Farma, who lists their offices on their official website. It’s in the Turkish, but Google Chrome can automatically translate. Doing so, we see they list only one factory, and its in Turkey. It is unlikely that they outsource production, being a relatively small pharmaceutical company, but there is always some risk of this, and it can be difficult to know or find out which company they contract to do so, depending on the country of origin.
Next, we’re going to perform a search of inspections databases. PharmacyCompass is a good start, as they provide inspections information collated from all major authorities worldwide. We got a hit! EDQM (European regulatory authority) says they’ve inspected them. If this manufactuerer were based in multiple countries, we’d want to check that the country in which the medicine is sourced from was where the site inspection took place (more on this later). One downside of PharmacyCompass is that their information may be out of date. In this case, it is. The inspection they list is from 2010.
Let’s head over to EudraGMDP, the inspections database for EDQM. Click GMP -> GMP Certificates to be brought to a search form. PharmacyCompass helpfully listed the information we’ll need to fill it in. Take the “EudraGMDR Key” value and plug it into the “EudraGMDP Site Reference Number” field. Select Turkey from the Country drop down. Make sure ‘Include Non-Compliance Report’ is checked and lets do our search!
I get four results. The latest inspection was in 2016. If we look back at the list of Kocak Farma’s sites, we see that the site address for these inspections matches their factory’s address. All of these documents are certifiates of inspection. If we view at the latest document, we’ll see that they seem to have passed. The inspection included formulation of tablets, packaging and quality control such as chemical analysis. That’s what we want to see! Had there been only a portion of these components of the process listed, we would have been tipped off that there may be more facilities involved that we had missed. Unfortunately, Turkey’s inspections authority does not appear to have a public database, which would have made tracking those sites down rather difficult.
This was an easy one. Other manufacturers may be larger and require evaluation of multiple sites.
A complex example
After evaluating my options, I can’t afford bicalutamide. I’ve heard there’s something cheaper for my anti-androgenic needs, so I start looking for spironolactone. A couple pharmacies stock it, and their price seems right (4nrx, UP)
Again, we’re told who made it and what country its sourced from. We look up Mylan on PharmacyCompass, but uh oh… what’s this? Mylan is a huge corporation with manufacturing plants all over the world. We click through all the inspection database summaries, but its confusing! Some of these addresses seem truncated, so it isn’t clear what country the sites are even in. To make matters worse, Mylan has entered countries over the years by buying up other companies. It might not even be Mylan who produced this medicine, but another company under their name but formally “doing business as” someone else. How do we make sense of this?
We could email the pharmacy’s support and ask for the product application records. We can also look that up ourselfes since New Zealand does have a public database of medicine registrations, Medsafe (Medicines and Medical Devices Safety Authority, part of their Ministry of Health). We know the trade name and the manufacturer. Let’s plug it in and see what we can find. This sheds some light on the situation. Now we see what compositing and manufacturing companies were involved and where they are. There are quite a few, spread from the United Kingdom, New Zealand, China to even Australia. Let’s dig in…
Zhejiang Shenzhou Pharmaceutical. PharmacyCompass doesn’t return results in the area of China that is listed for the site. Let’s try querying the FDA inspections database directly. We know the firm name and the country. Entering that gets us some results! Looks like they’ve passed.
Piramal Healthcare. Again, nothing on PharmacyCompass. FDA returns results. They received a VAI most recently. VAI stands for voluntary action indicated and NAI, no action indicated. Not great, but not inherently a huge deal. This classification does not jeopardize their ability to sell medicines in the country whose regulatory body issued the report. It bascially means the site meets the minimum requirements for GMP certification. A VAI that isn’t remedied does not necessarily lead to serious compliance violation during the next inspection, but it could, if new information is discovered that is related to the same issue, or the issue grows increasingly problematic.
If we’re feeling bold, we can check Wikileaks or a specifically crafted Google search to see if we can find a leaked report of some interest. They doesn’t return anything interesting, but Google does. Two annual financial reports (2017-2018, 2018-2019) are freely available on their website! These are long. Searching them for ‘inspections’ quicky turns up a relevant sentence, though. Both documents clearly state that they have cleared all FDA audits. They’re probably counting VAIs as clearances, and technically they are. Given their inspections history and the breadth of their operations, they have clearly flourished and take quality seriously, so I’ll end my curiosity inspired search here.
Finally, Alphapharm. PharmacyCompass doesn’t return the site we’re looking for. The FDA does, though. They’ve only received some VAIs in the past, but none recently. Looks like they too took quality seriously and addressed even these voluntary actions that the FDA recommended taking.
All in all, this makes me a little nervous, but since the inspection classification wasn’t an OAI, I would feel comfortable purchasing this product since we were able to look up every company involved with the production of this medicine.
A risky example
So far, we’ve evaluated sites that have passed their inspections without much ado. Let’s look at an example of inspections analysis that is concerning.
Our fictional transgirl needs estrogen! She’s not too familiar with the different formulations, and are only looking at prices right now. She finds one (ADC) and its very cheap compared to other products containing only estradiol. As it turns out, this is actually Estradiol valerate, as indicated by other pharmacies. It is mislabeled here as Estradiol, which can only be said if the product is hemihydrate.
The manufacturer is listed as Zydus Healthcare. PharmacyCompass has no inspections for this company. The FDA shows some companies vaguely matching Zydus, but no complete matches. EudraGMDP returns nothing at all. What’s going on here?
Let’s take to Google. Turns out Zydus was restructured under Cadila, but also they appear as Zydus Pharmaceuticals (US) specifically and Cadila Pharmaceuticals (IN) separately in the FDA database. They also bought a company called German Remedies according to their Wikipedia article. Which one of these manufacturered the drugs in question? It’d appear the acquisition doesn’t produce the same medicines, so they’re easily eliminated…
CDSCO is the main authority in India for approving drugs and enforcing GMP. I was unable to find any sort of pubically searchable database for inspections or registrations though. The closest I got was a licensing portal for submitting and reviewing your own application. Unfortunately, the warnings from regulatory authorities about overseas drugs potentially being subpar is not scare mongering, as you can see. Some of these countries not only house sites with problematic practices, but their governments do not provide tools with which to find out what precisely might be worrisome.
The packaging may hold the key. ADC’s packaging image is woefully out of date, but a quick image search turns up some with recent lot dates.
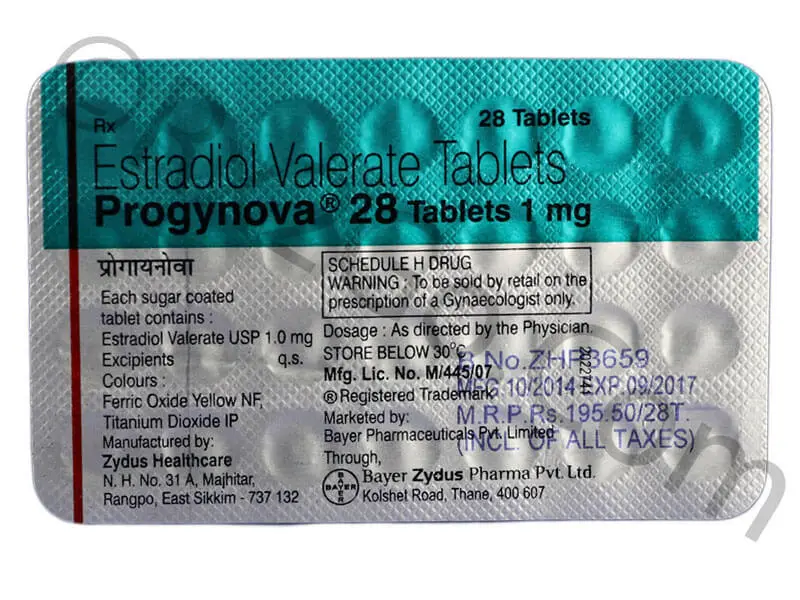
We can see that this Zydus Healthcare Ltd. is still listed as the company responsible for production, so they’re still doing business under that name up until fairly recently. It also separately denotates this from the marketing entity. This one is a few years old. The more recent is this one from 2018:
It’s low quality but if you squint really hard it essentially says the same thing. All packaging is consistent in this product being manufacturered in India at that the same exact location, which raises our confidence that there aren’t sites we missed…
But the FDA’s inspections database doesn’t list this company! How can we find it? This isn’t always the case, but the parent company’s website might list it. Turns out they do, and even helpfully name each site by function. This is what we need to know! One of them, under Sakkin Formulations Mfg. shows the business name and location on the packaging. Finally some answers! I’d infer that this product is not sold in the US by the lack of it ever having been inspected. This is confirmed by a search of the FDA approved drugs database, which lists only injectable estradiol valerate. This seriously complicates our research and introduces a pretty big unknown into what risk analysis we can make. The best we can do is look at other production facilities and loosely base our confidence abstractly on them instead.
They have received many VAIs and at least one OAI in the past under the names Zydus and Cadila. Super troubling! Four inspections occurred in 2019; two resulted in VAI and another in OAI, yet all inspections in 2020 have been fine so far. Out of all their inspections in the past, they’ve received eigteen VAIs and five OAIs. This is looking pretty grim.
We can search for any warning letters that the FDA issued to them related to this event. Cadila received two such letters, both issued in 2015 (here, and here). These letters lay out several conerns discovered by an inspector, and detail failure to remediate those same concerns. Compliance violations resulting in pharmaceutical impurities, inadequate data retention and negligent review of QA data were noted and went uncorrected. As a result, the FDA warned them that until they were convinced that remediations had taken place, they may decline acceptance of medications from the site in question into the US.
For the OAI at a synthesis site in 2019, the problem was not as bad. It was classified as not posing a risk to health, but revealed some troubling logic in the company’s response to the FDA.
Your firm’s review concluded that the significant cross-contamination identified by your firm does not represent a risk to patients.
Your response is insufficient. Your response stated that any potential residue that enters the (b)(4) and contaminates the next drug product can produce a nearly uniform distribution in the (b)(4) and that (b)(4) steps minimize localization of carryover residue. Your rationale is not scientifically sound in that cross-contamination cannot be assumed to be uniformly distributed.
In addition, your response described failure modes that may have contributed to the accumulation of residues in the (b)(4). But you failed to explain when the cross-contamination involving numerous products started and why it had not been detected. Your response also stated that testing for cross-contamination in the products provides good assurance that any carryover is detected. However, reserve sample testing alone is insufficient to mitigate associated risks. The extent of the cross-contamination found suggests a lack of assurance that products meet appropriate standards for identity, quality, purity and safety.
They clearly have an interest in improving, though it appears to be in part motivated by show of force. A recall was mandated and carried out. Since this was a lower class of OAI, the FDA did not block imports from this facility, but warned that if they didn’t correct the problem, that they may in the future. The same facility was inspected several months later and was cleared.
This manufacturer has multiple sites with a long history of questionable manufacturing practices. A couple of them have failed inspections in recent times, one of which pretty spectacularly. Between this track record and the unknown policies at the site which actually produces the product in question, I’d be uncomfortable purchasing this product. If you’re willing to do more work, you may be able to enquire with the pharmacy for the lot number of the medicine, and then with the manufacturer’s regional office near you to definitively hear which site the lot was produced at and request registration or inspection data. Whether or not they’d comply with such a request is another matter.
Do what you’re comfortable with
There have been times when the medical establishment has failed me in providing timely appointments to review my medication. I’ve purchased from greynet pharmacies when I’ve felt confident in making simple, safe adjustments to prescriptions, and to stockpile hormones for my own treatment. I do research like this for my own peace of mind in these circumstances. It provides insight into the business practices of companies in regions that have poor oversight and known quality control issues. People aren’t perfect despite their best intentions, and capitalism has a way with creating perverse incentives. Knowing this, it makes me feel better to see with my own eyes and through critical thought that I am making the best decision with respect to my health. I think it is important to give others the option to make similar decisions like this, and hope this essay provided a solid base by which this can be achieved, if desired.
« The odds of SARS2 infection ✧ Simple self-hosted file sharing »